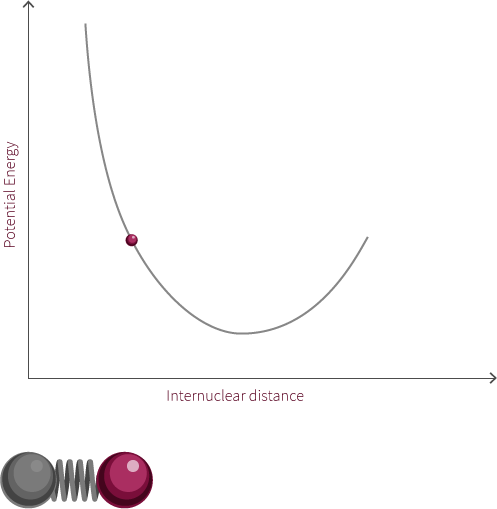
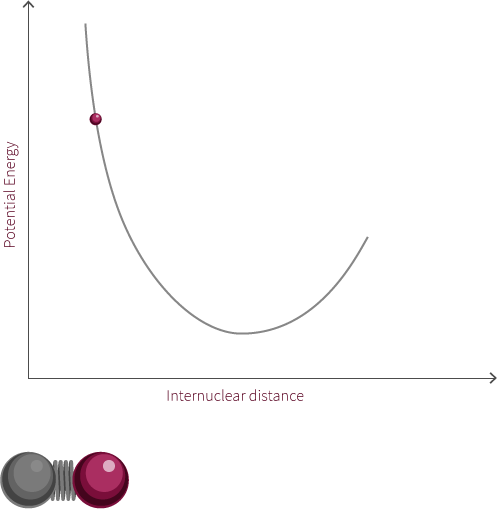
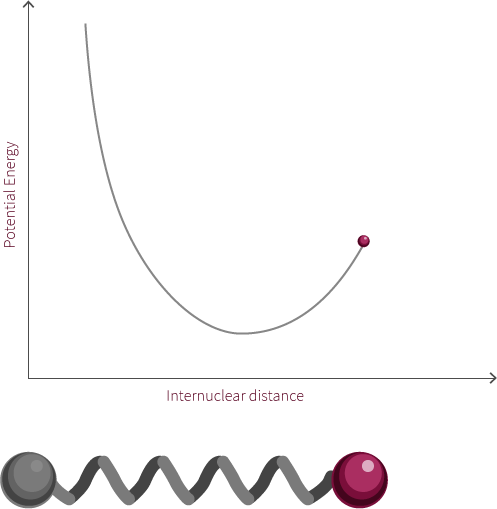
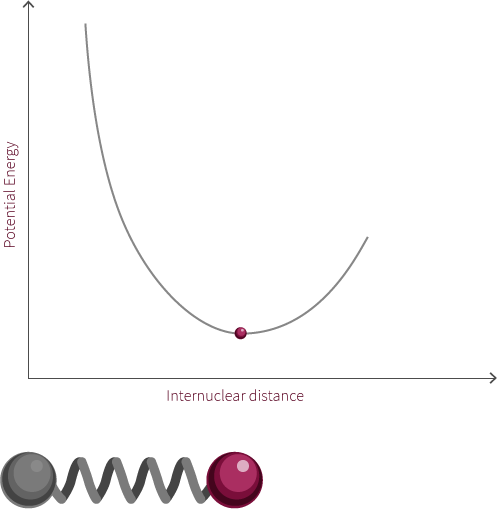
We can approximate the energy of our diatomic using the Morse Potential we have encountered earlier (we are ignoring the quantum nature of the molecule for now).
We have simultaneously shown the potential energies of the π and π* orbitals below. If an electron were to transfer from the π to the higher energy π* orbital, it is said to have been promoted to an excited state. This process is known as molecular excitation. We can determine the change in energy by taking the difference in potential energy before and after the excitation event.
Morse Potential
Molecular excitation is not a random event and requires strict conditions in order to occur. Before we can learn more about molecular excitation, we must first understand the energy and other properties of our diatomic system.
Just like two balls on a spring, the atoms are stretched and compressed along its bond. However, unlike the spring, the energy of this system is modelled by the Morse potential like the electrostatic spheres model.
Spring, Pi, Spheres
The Morse potential is modelled by the following expression:
Morse Equation
Grab the atom on the right and try moving it left and right. What happens to the energy as you do so? What happens if you pull the bond much further to the right? Why does the system look like this now? Why does the energy change in this way?