Another contribution to the quantized energy of the diatomic molecule is its rotational energy. In the diatomic system, there exists a single rotational mode, similar to how a single vibrational mode exists for the diatomic molecule. Also, just like the vibrational state, a more complicated system (ie. a molecular with more than two atoms) would have multiple rotation states.
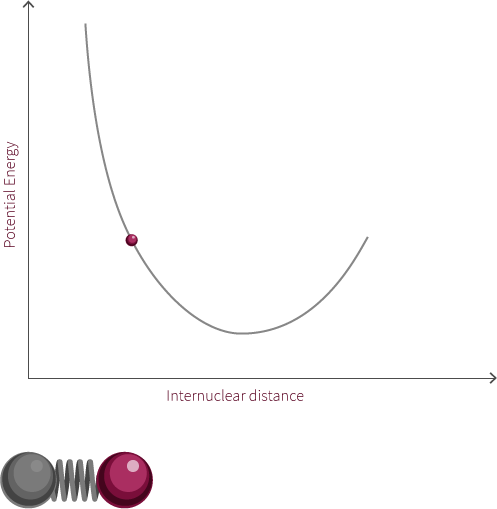
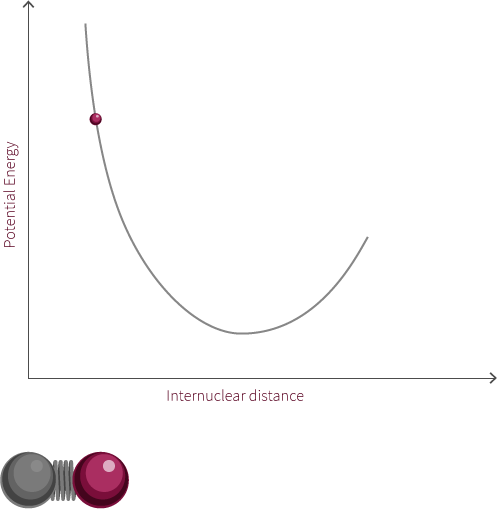
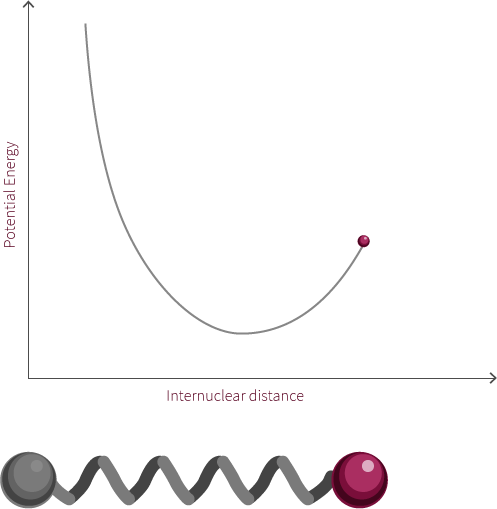
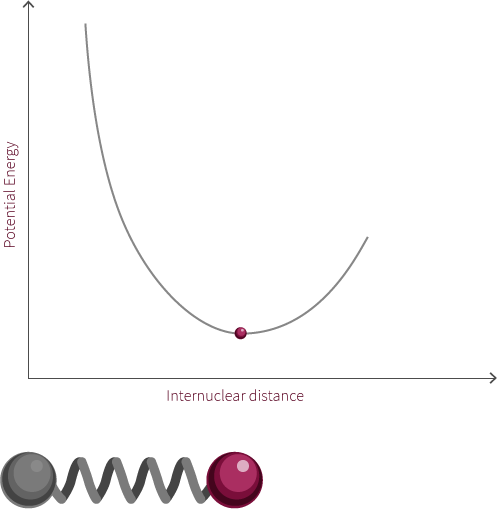
Morse Potential
The observed rotation is due to the specific angular momentum of the diatomic molecule. Quantum mechanics is used to determine the allowed angular momentum states. The magnitude of the rotational energy is considerably smaller than the vibrational energy, as can be observed in the diagram above.
For our diatomic molecule only a single rotational mode available is available and it occurs about an axis perpendicular to the bond axis. The energy of the rotational mode is directly related to its angular momentum. Molecular rotation takes place at a nanosecond time scale (~10-9s).
rotation axis
Molecules can gain and lose energy from their surroundings which can change both the systems vibrational or rotational energy. Another source of energy is electromagnetic radiation. In fact, electromagnetic radiation is capable of promoting electrons to an excited state.
In the system to the left, you can alter both the current rotational mode and vibrational state by clicking on the selector, clicking on the π bond, or clicking on the vibration/rotation energy state. You can also switch molecular vibration and rotation 'on' or 'off'. What do you notice when you increase the rotational mode? What would you expect to happen as a molecule loses energy?