Our exploration into molecular excitation begins with a description of simple harmonic oscillators. A simple harmonic oscillator displays a very particular type of periodic motion called simple harmonic motion. A common example of a simple harmonic oscillator is a spring that oscillates between a stretched and compressed position.
spring oscillation
Physicists often use springs as a model to help explain complicated systems like the motion of fluids or the behaviour of atoms in a bond. Simple harmonic motion is useful for starting to understand concepts in quantum chemistry. More complicated descriptions of atomic bonds will be addressed later.
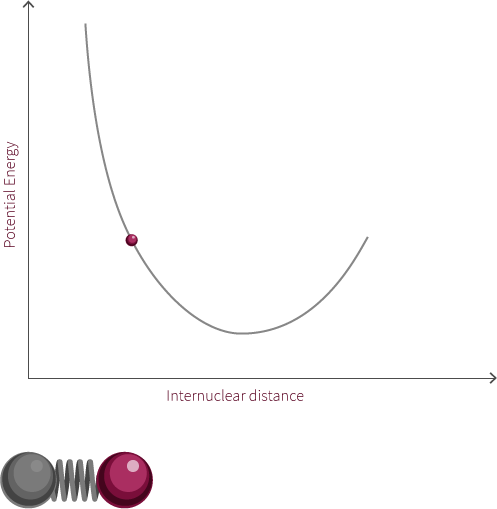
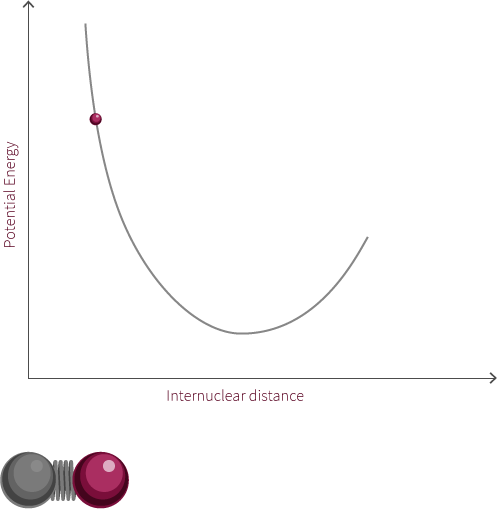
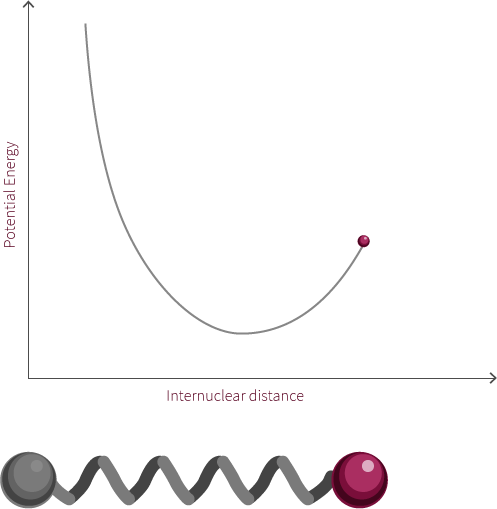
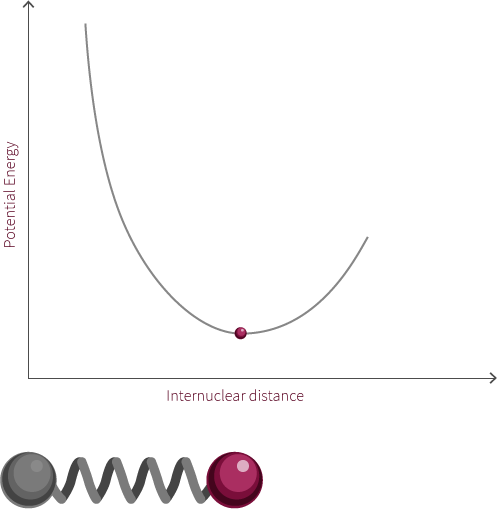
An example of an everyday harmonic oscillator is a spring. A spring at rest, meaning it is neither compressed nor stretched, is said to be at its equilibrium position. A spring will resist being moved from this position because it is currently at its lowest energy configuration. When the spring is stretched or compressed from its equilibrium position, the spring increases in its potential energy. In the diagram below, x represents the distance the spring is from its equilibrium position.
spring equilibrium
Try stretching and compressing the spring to the left. Observe the relationship between the distance from the springs equilibrium position and changes to its potential energy. What else do you notice? What happens to the motion over time? Try stretching the spring as far to the right as possible. What happens?