Feminizing Hormone Therapy
The goal of hormone therapy in trans women is to reduce the endogenous effects of testosterone such as coarse body hair and facial hair; and to induce female secondary sex characteristics such as breast and hip development. Physiologically, this requires a suppression of endogenous androgens and the addition of estrogen.
Quick reference guide for feminizing hormone therapy
Hormonal agents
Anti-androgens
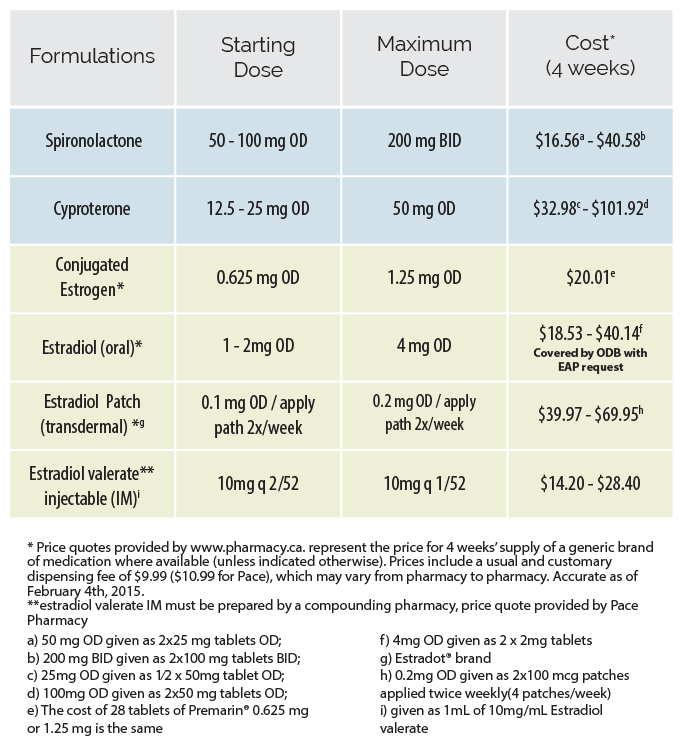
Spironolactone has traditionally been used preferentially as it was thought to have a superior safety profile. This practice has recently come into question as it has been noted that adequate anti-androgen effects are achievable at lower doses of cyproterone at which adverse effects are less likely. Thus the choice of anti-androgen should be made individually for each client based on their medical history and preference regarding respective side effect profiles.
Following orchiectomy (+/- vaginoplasty), most trans women will not require androgen suppression. The androgen-blocker can be tapered over the course of 4-6 weeks.
Estrogen
Estrogen acts directly on estrogen receptors to initiate feminization. It is usually the focus of hormonal transition for trans women. The starting dose of estrogen can be maintained for 1-2 months, after which a dose increase can be considered barring any concerning effects. In clients over 50 years old who have been on estrogen for several years, doses may be reduced to those administered to post-menopausal cis women (ie. 0.025 – 0.05 mg patch).
View formulations, recommended doses and costs of anti-androgen and estrogen
Expected effects
What to expect from a regimen consisting of an anti-androgen and estrogen
The degree and rate of physical effects is dependent on the dose and route of administration, as well as client-specific factors such as age, genetics, body habitus and lifestyle.
Physical changes related to androgen blockade and estrogen may take months to appear and are generally considered to be complete after 2-3 years on hormone therapy. Feminizing therapy does not affect the pitch of the voice in trans women. Some clients may obtain benefit from voice therapy with a qualified and supportive speech and language therapist who can work with the client to modify their vocal characteristics.
Effects and expected time course Download
Risk mitigation
Absolute contraindications
- Unstable ischemic cardiovascular disease
- Estrogen-dependent cancer
- End stage chronic liver disease
- Psychiatric conditions which limit the ability to provide informed consent
- Hypersensitivity to one of the components of the formulation
Precautions and risk mitigation Download
Several pre-existing medical conditions and risk factors may increase the risks associated with estrogen administration. When these are present, a careful evaluation of risks and benefits should be completed and fully discussed with the client.
Precautions in red impart moderate to high risk of an adverse outcome without risk mitigation.
Select area of concern below
Neurologic
More information on seizure disorders and anticonvulsant therapy on page 17 of the full Protocols
| Risk factors | How to minimize risks |
|---|---|
| Cerebrovascular disease | Consider referral to neurology, ensure optimal medical management (including prophylactic anticoagulation) and aggressive risk factor optimization, use transdermal route of administration +/- lower dose |
| Severe refractory or focal migraine | Consider referral to neurology, consider daily migraine prophylaxis, ensure all other cerebrovascular risk factors are optimized, consider transdermal route of administration |
| Seizure disorders | Consider referral to neurology, consult with a pharmacist re: impact of estrogen interaction with anticonvulsant medication |
| History of benign intracranial hypertension | Consider referral to neurology/neurosurgery |
Endocrine
| Risk factors | How to minimize risks |
|---|---|
| Hyperprolactinemia | Refer to endocrinology, defer initiation until etiology determined, manage based on etiology. More information on hyperprolactinemia/Prolacinoma on page 17 of the full Protocols |
| Marked hypertriglyceridemia | Identify and address barriers to optimal lipid control, refer to dietician, minimize alcohol consumption, consider anti-lipemic pharmacologic therapy, consider endocrinology referral, encourage deferral of estrogen until controlled, consider transdermal route of administration |
| Uncontrolled diabetes | Identify and address barriers to optimal glycemic control, refer to dietician, encourage lifestyle modification, initiate antiglycemic agent(s), encourage deferral of estrogen until controlled, consider cardiac stress test, consider transdermal route of administration. |
| Metabolic syndrome | Dietary and medical management of component disorders, encourage deferral until components adequately managed, consider cardiac stress test, consider transdermal route of administration |
Cardiovascular
More information on cardiovascular and cerebrovascular disease on page 17 of the full Protocols
| Risk factors | How to minimize risks |
|---|---|
| Stable ischemic cardiovascular disease | Consider referral to cardiology, ensure optimal medical (including prophylactic anticoagulation) and/or surgical management as indicated, aggressive risk factor optimization, use transdermal route of administration +/- lower dose |
| Other cardiac diseases | Consider referral to cardiology |
| Uncontrolled high blood pressure | Identify and address barriers to optimal BP control, use spironolactone as antiandrogen, add additional antihypertensives as needed (avoid ACEs/ARBs with spironolactone), encourage deferral of estrogen until controlled, consider cardiac stress test, consider transdermal route of administration |
Hepatic
More information on liver/gallbladder effects on page 17 of the full Protocols
| Risk factor | How to minimize risks |
|---|---|
| Hepatic dysfunction | Dependent on etiology, eg. minimize alcohol consumption, weight loss in NAFLD, consider referral to hepatology/GI, use transdermal or injectable route of administration |
Oncologic
More information on breast cancer on page 17 of the full Protocols
| Risk factors | How to minimize risks |
|---|---|
| Strong family history of breast cancer | Refer to genetics/familial breast cancer program for further risk stratification and BRCA1/2 testing as indicated |
Hematologic
| Risk factors | How to minimize risks |
|---|---|
| Personal or family history of porphyria (rare) | Consider referral to porphyria clinic or internist with experience in porphyria |
| Personal history of deep vein thrombosis (DVT) or pulmonary embolism (PE) | Identify and minimize co-existent risk factors, consider prophylactic anti-coagulation, consider referral to hematology, use transdermal route of administration +/- lower dose |
| Family history of abnormal clotting | Consider referral to hematology, rule out genetic clotting disorder, consider prophylactic anticoagulation, use transdermal route of administration |
Respiratory
| Risk/Precaution | How to minimize risks |
|---|---|
| Smoker | Encourage and support smoking cessation, offer NRT and/or bupropion/varenacline, or negotiate a decrease in smoking, consider lower dose, consider cardiac stress test, use transdermal route of administration |
Monitoring strategies
Standard monitoring of estrogen administration should be employed at baseline, 1, 3, 6, and 12 months. This should include a functional inquiry, targeted physical exam, bloodwork, and health promotion/ disease prevention counselling.
Testosterone level may be the most useful test for monitoring in trans women; for many clients, the goal will be to achieve the suppression of testosterone into the female range. That said, the client may have clinically relevant results without total suppression of testosterone because of androgen blockade, which is not easily measured. Estradiol levels are of variable utility in monitoring feminizing therapy given the wide cyclical variation in cis women. Most clients attain considerable feminization at estradiol levels between 200-500 pmol/L. According to the Endocrine Society Guidelines, serum estradiol levels should not exceed the mean daily level for cis women (approximately 700 pmol/L).
Hormone Monitoring Summary Download
Click on one of the tabs to find out standard monitoring suggestions at baseline, 1, 3 and 6 months.
Review with client:
-
Contraindications and precautions
-
Old records
-
Mental health
- Screen for depressive symptoms (including suicidality) and anxiety disorders
- Inquire re: symptoms of hypomania, mania, or psychotic symptoms
- Inquire re: current level of gender dysphoria and body image
- Screen for disordered eating
- Assess client interest in surgical treatments if not accessed
- Inquire re: libido/changes in libido
-
Education / Lifestyle Counselling
- Review healthy eating and general nutrition
- Adequate Calcium Intake – ensure a minimum intake of 1200 mg of Calcium daily (total: diet + supplements)
- Adequate Vitamin D – ensure 1000 IU of vitamin D daily
- Hormone Adherence – missed doses of estrogen impacts bone health if post-orchiectomy, while extra doses may lead to risks associated with supraphysiologic levels of estrogen
- Regular, moderate physical activity – encourage weight-bearing exercise for osteoporosis prevention as well as aerobic exercise
- Alcohol and other substances – inquire re: problematic use of substances including alcohol, cannabis, cocaine, opioids, hallucinogens, ketamine, ecstasy, and non-prescribed hormones; estrogen affects the metabolism of alcohol by the liver thus we suggest using the same safe-drinking guidelines for trans women as for cis women (see Canada’s Low-risk Alcohol Drinking Guidelines)
- Smoking – cessation, stages of readiness, etc.
- STI Prevention – transgender women may be at high risk of STIs depending on behavioural factors; safer sex counselling and frequent screening (i.e. every 3 months) for those at high risk is imperative (for client-centred handout materials, see Brazen: a Trans women’s Safer Sex Guide)
- Review the signs and symptoms of Deep vein thrombosis (DVT) and Pulmonary Embolism (PE) and advise immediate medical attention should these occur
-
Psychosocial
- An effort should be made to assess the impact of transition/trans identity on employment, housing, family, relationships, and economic wellbeing
- Social Supports – specific attention should be given to assessing the extent of a client’s social supports, creating an opportunity to suggest additional resources if needed
- Name change/identification – assess client need/ desire to change name and/or gender marker on identification and offer support for this process (see template letters and RHO fact sheets in the resources section)
-
Bone health
-
Health maintenance
- Immunization history
- STI screening, HIV risk assessment and screening
- TB skin test
Examinations/Investigations:
-
Full Physical Exam
-
Measurements
- height, weight, waist & abdo circ., +/- breast, hips as per client preference
-
EKG / cardiac stress test
- EKG for clients over 40
- EKG and cardiac stress test if client has additional risk factors
Bloodwork:
-
CBC
-
ALT/AST (if restricted in ordering OHIP-covered AST levels, ALT alone may be used to screen for liver dysfunction)
-
Creatinine/Lytes/Urea
-
Testosterone (+/- Estradiol)
-
Fasting Glucose
-
LDL/HDL/TG
-
Prolactin
-
LH (Elevated LH post-gonadectomy may have implications regarding bone mineral density - See Full Protocols for Osteoporosis and BMD Screening)
Review with client:
-
Hormone effects
-
Spontaneous erections
-
Mental health
- Screen for depressive symptoms (including suicidality) and anxiety disorders
- Inquire re: symptoms of hypomania, mania, or psychotic symptoms
- Inquire re: current level of gender dysphoria and body image
- Screen for disordered eating
- Assess client interest in surgical treatments if not accessed
- Inquire re: libido/changes in libido
-
Education / Lifestyle Counselling
- Review healthy eating and general nutrition
- Adequate Calcium Intake – ensure a minimum intake of 1200 mg of Calcium daily (total: diet + supplements)
- Adequate Vitamin D – ensure 1000 IU of vitamin D daily
- Hormone Adherence – missed doses of estrogen impacts bone health if post-orchiectomy, while extra doses may lead to risks associated with supraphysiologic levels of estrogen
- Regular, moderate physical activity – encourage weight-bearing exercise for osteoporosis prevention as well as aerobic exercise
- Alcohol and other substances – inquire re: problematic use of substances including alcohol, cannabis, cocaine, opioids, hallucinogens, ketamine, ecstasy, and non-prescribed hormones; estrogen affects the metabolism of alcohol by the liver thus we suggest using the same safe-drinking guidelines for trans women as for cis women (see Canada’s Low-risk Alcohol Drinking Guidelines)
- Smoking – cessation, stages of readiness, etc.
- STI Prevention – transgender women may be at high risk of STIs depending on behavioural factors; safer sex counselling and frequent screening (i.e. every 3 months) for those at high risk is imperative (for client-centred handout materials, see Brazen: a Trans women’s Safer Sex Guide)
- Review the signs and symptoms of Deep vein thrombosis (DVT) and Pulmonary Embolism (PE) and advise immediate medical attention should these occur
-
Psychosocial
- An effort should be made to assess the impact of transition/trans identity on employment, housing, family, relationships, and economic wellbeing
- Social Supports – specific attention should be given to assessing the extent of a client’s social supports, creating an opportunity to suggest additional resources if needed
- Name change/identification – assess client need/ desire to change name and/or gender marker on identification and offer support for this process (see template letters and RHO fact sheets in the resources section)
Examinations/Investigations:
-
Measurements
- blood pressure, weight, waist & abdo circ.
-
Extremity Exam
-
Abdominal exam including liver palpation
-
Immunizations
- Vaccinate for Hep A & B, Td and Pneumovax as indicated, Consider HPV vaccine
Bloodwork:
-
CBC
-
ALT/AST (if restricted in ordering OHIP-covered AST levels, ALT alone may be used to screen for liver dysfunction)
-
Creatinine/Lytes/Urea
-
Testosterone (+/- Estradiol)
-
Prolactin
-
LH (Elevated LH post-gonadectomy may have implications regarding bone mineral density - See Full Protocols for Osteoporosis and BMD Screening)
Review with client:
-
Hormone effects
-
Spontaneous erections
-
Mental health
- Screen for depressive symptoms (including suicidality) and anxiety disorders
- Inquire re: symptoms of hypomania, mania, or psychotic symptoms
- Inquire re: current level of gender dysphoria and body image
- Screen for disordered eating
- Assess client interest in surgical treatments if not accessed
- Inquire re: libido/changes in libido
-
Education / Lifestyle Counselling
- Review healthy eating and general nutrition
- Adequate Calcium Intake – ensure a minimum intake of 1200 mg of Calcium daily (total: diet + supplements)
- Adequate Vitamin D – ensure 1000 IU of vitamin D daily
- Hormone Adherence – missed doses of estrogen impacts bone health if post-orchiectomy, while extra doses may lead to risks associated with supraphysiologic levels of estrogen
- Regular, moderate physical activity – encourage weight-bearing exercise for osteoporosis prevention as well as aerobic exercise
- Alcohol and other substances – inquire re: problematic use of substances including alcohol, cannabis, cocaine, opioids, hallucinogens, ketamine, ecstasy, and non-prescribed hormones; estrogen affects the metabolism of alcohol by the liver thus we suggest using the same safe-drinking guidelines for trans women as for cis women (see Canada’s Low-risk Alcohol Drinking Guidelines)
- Smoking – cessation, stages of readiness, etc.
- STI Prevention – transgender women may be at high risk of STIs depending on behavioural factors; safer sex counselling and frequent screening (i.e. every 3 months) for those at high risk is imperative (for client-centred handout materials, see Brazen: a Trans women’s Safer Sex Guide)
- Review the signs and symptoms of Deep vein thrombosis (DVT) and Pulmonary Embolism (PE) and advise immediate medical attention should these occur
-
Psychosocial
- An effort should be made to assess the impact of transition/trans identity on employment, housing, family, relationships, and economic wellbeing
- Social Supports – specific attention should be given to assessing the extent of a client’s social supports, creating an opportunity to suggest additional resources if needed
- Name change/identification – assess client need/ desire to change name and/or gender marker on identification and offer support for this process (see template letters and RHO fact sheets in the resources section)
Examinations/Investigations:
-
Measurements
- blood pressure, weight, waist & abdo circ.
- breast and hips as per client preference
-
Extremity Exam
-
Abdominal exam including liver palpation
Bloodwork:
-
CBC
-
ALT/AST (if restricted in ordering OHIP-covered AST levels, ALT alone may be used to screen for liver dysfunction)
-
Creatinine/Lytes/Urea
-
Testosterone (+/- Estradiol)
-
Prolactin
-
LH (Elevated LH post-gonadectomy may have implications regarding bone mineral density - See Full Protocols for Osteoporosis and BMD Screening)
Review with client:
-
Hormone effects
-
Spontaneous erections
-
Mental health
- Screen for depressive symptoms (including suicidality) and anxiety disorders
- Inquire re: symptoms of hypomania, mania, or psychotic symptoms
- Inquire re: current level of gender dysphoria and body image
- Screen for disordered eating
- Assess client interest in surgical treatments if not accessed
- Inquire re: libido/changes in libido
-
Education / Lifestyle Counselling
- Review healthy eating and general nutrition
- Adequate Calcium Intake – ensure a minimum intake of 1200 mg of Calcium daily (total: diet + supplements)
- Adequate Vitamin D – ensure 1000 IU of vitamin D daily
- Hormone Adherence – missed doses of estrogen impacts bone health if post-orchiectomy, while extra doses may lead to risks associated with supraphysiologic levels of estrogen
- Regular, moderate physical activity – encourage weight-bearing exercise for osteoporosis prevention as well as aerobic exercise
- Alcohol and other substances – inquire re: problematic use of substances including alcohol, cannabis, cocaine, opioids, hallucinogens, ketamine, ecstasy, and non-prescribed hormones; estrogen affects the metabolism of alcohol by the liver thus we suggest using the same safe-drinking guidelines for trans women as for cis women (see Canada’s Low-risk Alcohol Drinking Guidelines)
- Smoking – cessation, stages of readiness, etc.
- STI Prevention – transgender women may be at high risk of STIs depending on behavioural factors; safer sex counselling and frequent screening (i.e. every 3 months) for those at high risk is imperative (for client-centred handout materials, see Brazen: a Trans women’s Safer Sex Guide)
- Review the signs and symptoms of Deep vein thrombosis (DVT) and Pulmonary Embolism (PE) and advise immediate medical attention should these occur
-
Psychosocial
- An effort should be made to assess the impact of transition/trans identity on employment, housing, family, relationships, and economic wellbeing
- Social Supports – specific attention should be given to assessing the extent of a client’s social supports, creating an opportunity to suggest additional resources if needed
- Name change/identification – assess client need/ desire to change name and/or gender marker on identification and offer support for this process (see template letters and RHO fact sheets in the resources section)
Examinations/Investigations:
-
Measurements
- blood pressure, weight, waist & abdo circ.
- breast and hips as per client preference
-
Extremity Exam
-
Abdominal exam including liver palpation
Bloodwork:
-
CBC
-
ALT/AST (if restricted in ordering OHIP-covered AST levels, ALT alone may be used to screen for liver dysfunction)
-
Testosterone (+/- Estradiol)
-
Prolactin
-
LH (Elevated LH post-gonadectomy may have implications regarding bone mineral density - See Full Protocols for Osteoporosis and BMD Screening)
CBC
ALT/AST (if restricted in ordering OHIP-covered AST levels, ALT alone may be used to screen for liver dysfunction)
Testosterone (+/- Estradiol)
Prolactin
LH (Elevated LH post-gonadectomy may have implications regarding bone mineral density - See Full Protocols for Osteoporosis and BMD Screening)
Long-term preventive care
Trans women maintained on feminizing hormone therapy have unique preventive care needs and recommendations.
Long-term care of trans women on feminizing hormone therapy should involve (at least) annual preventive care visits. An Adaptive Preventive Care Checklist with accompanying explanations for trans-specific recommendations can be accessed below.
-
Adapted Preventive Care Checklist for Trans Women
-
Accompanied Explanations for Trans-specific Recommendations
Preventive care screening procedures
Physical examinations that involve intimate body parts are discomforting to anyone. While many trans people are comfortable with their bodies others may experience body dysphoria. Some may be very uncomfortable with physical examinations or be reluctant to acknowledge or touch their own genitals.
View tips on increasing client comfort before, during and after exams
Provide care based on organs present
It is best to base routine screening on the presence or absence of body parts. Sex assigned at birth and gender identity are separate things. Some women have pensises, some men have vaginas. Refrain from calling body parts ‘male’ or female’. Instead use technical terms or ask client what they prefer to call their body parts. Organs present should receive routine and preventive care.
Click on one of the tabs to find out routine care and screening suggestions.
Osteoporosis and Bone Mineral Density Screening
Indications for BMD screening
- Clients over 65 years old
- Clients over 50 years old and with higher risk for osteoporosis
- Clients with any other risk factors for osteoporosis or bone loss (glucocorticoid therapy, previous fractures, family history of osteoporosis)
- Agonadal clients with eleveated LH levels
- Clients at any age having undergone orchiectomy and having been off exogenous hormones for any significant length of time
- Clients on anti-androgen therapy for a significant length of time without co-administration of exogenous estrogen
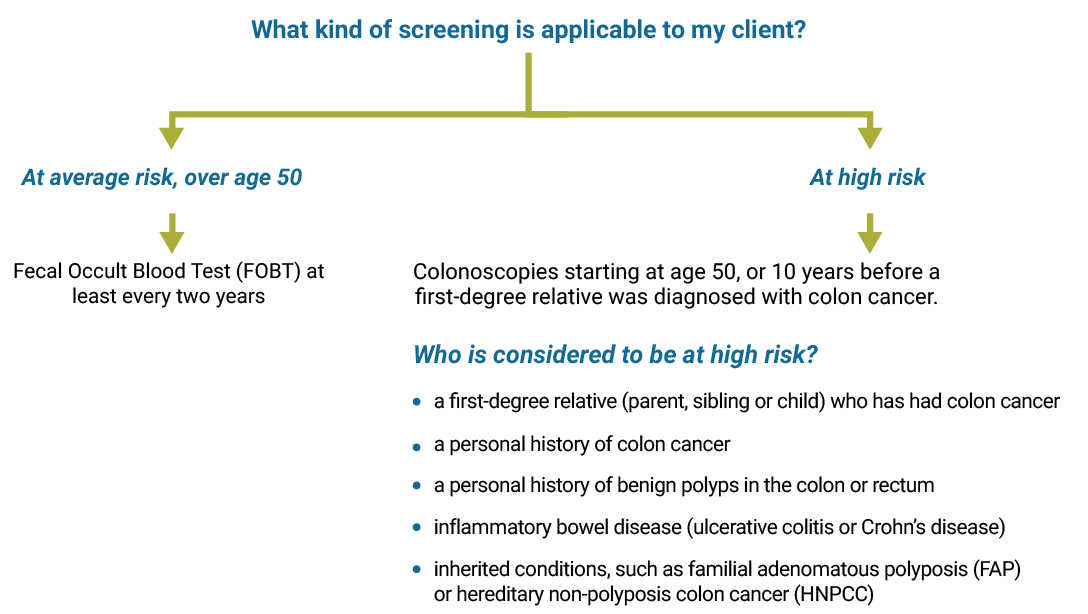
Colon cancer screening
Use the diagram below to find out when and what type of colon cancer screening is recommended.
Cervical cancer screening
Use the diagram below to find out what type of cervical cancer screening is recommended.

Prostate exam
The risk of prostate cancer is not increased by estrogen use, in fact it is reasonable to assume that the risk is significantly decreased by the associated androgen deprivation. Although rare, there have been cases of prostate cancer reported in trans women, generally occurring in those who started hormone therapy after the age of 50.1, 2 It is important to note that estrogen will lower PSA values even in the presence of prostate cancer, thus impacting its utility in this population. Routine PSA screening is not recommended in trans women in the absence of significant risk factors. There is little evidence to support a role for annual DRE in prostate cancer screening; however, it may be considered according to a provider’s routine practice with cis men. In clients who have undergone vaginoplasty, the prostate remains in situ and may be palpated anteriorly via digital vaginal exam in a gender affirming lithotomy position.
Sources
- Gooren LJ, Giltay EJ, Bunck MC. Long term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008; 93(1):19-25.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008; 159(3):197-202.