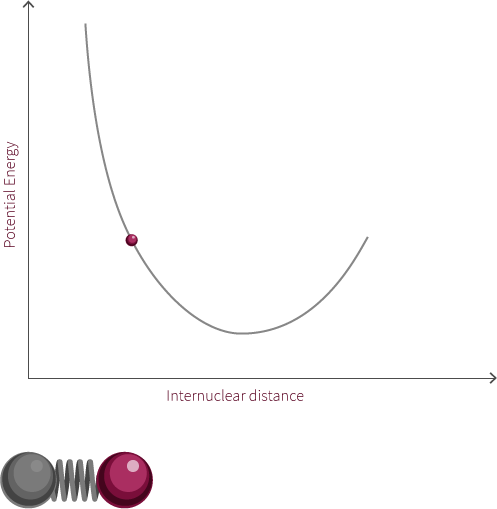
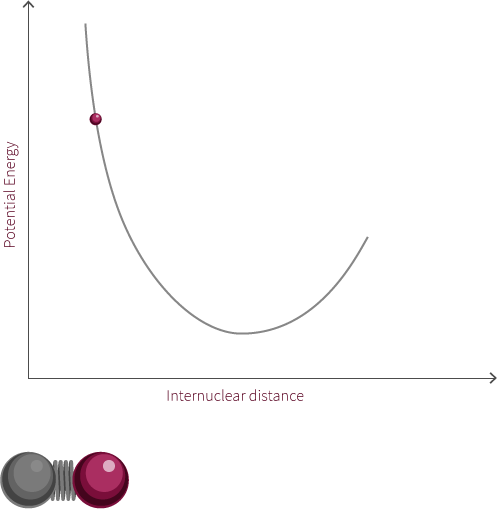
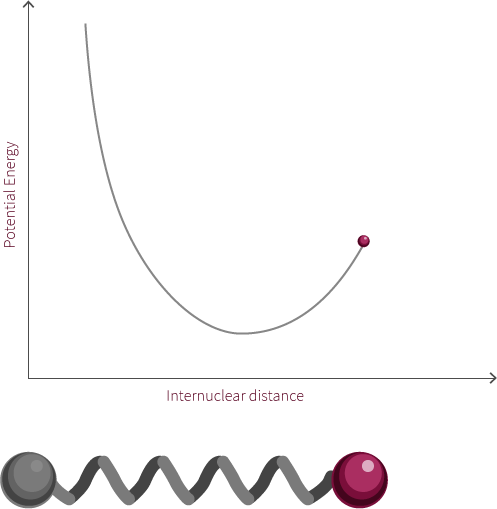
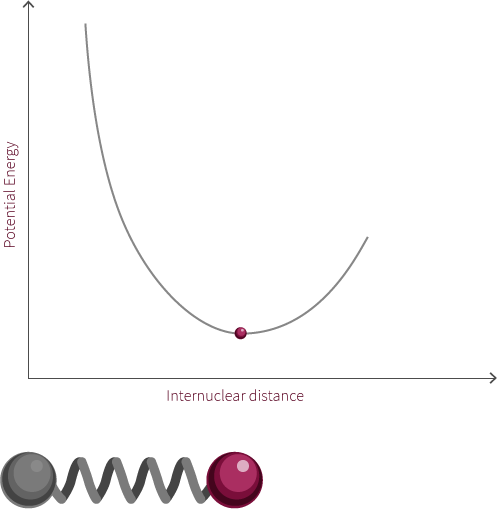
To move forward, we will need to switch our model from the two electrostatic spheres to a diatomic molecule.
diatomic molecule
Visualized above is an idealized diatomic system connected by a double bond. The double bond is composed of two bonds, the σ bond and the π bond.
pi orbitals
Shown above is the π molecular orbital that corresponds to the π bond. There are other orbitals involved in this system. Be sure to explore sections on molecular orbitals to brush up on your knowledge on molecular orbitals.
Our simple diatomic molecule has a low molecular weight and a double bond (the second bond in the double bond is referred to as a π bond). Briefly, the π bond is formed due to an interaction between the atomic p-orbitals of each atom. The π bonding molecular orbital is formed along with a π* anti-bonding molecular orbital.
pi orbitals
The system to the left has been simplified to show only the π and π* molecular orbitals when the atoms are close enough for bonding to take place. The atomic p-orbitals are shown at a distance where they are no longer interacting and forming a bond. An electron configuration energy diagram shows the approximate changes to the energy of the system.
You can click on the orbitals or energy levels on the energy diagram to see how the energy of the electron changes depending on what orbital is occupied. What other orbitals would you expect to find in a molecule with a double bond?